Consider the following energy-level diagram for a particular electron in an atom. 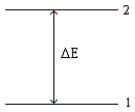 Based on this diagram,which of the following statements is incorrect?
Based on this diagram,which of the following statements is incorrect?
Definitions:
Require Adaptation
The necessity for adjustments or changes in behavior, processes, or structures in response to new conditions or environments.
Life Events
Major events in a person’s life that require change or adaptation.
Unpleasant
Causing discomfort, distress, or aversion; the opposite of pleasant.
Stressful Occupations
Jobs or professions that impose high levels of stress due to factors like tight deadlines, high responsibility, danger, or the need for long hours.
Q4: Which of the following is best described
Q10: What is the final concentration of HCl
Q28: What is the standard enthalpy of formation
Q29: One step in the isolation of pure
Q34: Rank the following in order of increasing
Q41: In basic solution the chromate ion,CrO<sub>4</sub><sup>2-</sup>,can be
Q49: Which of the following species has(have)a bond
Q52: Which of the following is a correct
Q81: A particular gas exerts a pressure of
Q94: The following representation of an atom is