Given the molecular orbital diagram for dinitrogen (N2) excluding the K shells below and assuming all species have a similar ordering of their MO's,which of the following would be expected to be diamagnetic? 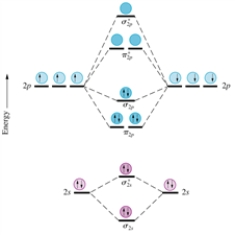
Definitions:
Payee
The individual or entity to whom a payment is to be made or is made.
Certificate of Deposit
A savings certificate entitling the bearer to receive interest, which has a fixed maturity date and specified fixed interest rate.
Acknowledgement
The act of recognizing or admitting the truth, validity, or legality of something.
Maker
A person who makes or executes an instrument. The signer of an instrument.
Q25: Which of the following statements is true
Q26: The reaction A → products is first-order
Q39: Which of the following statements concerning ozone
Q47: What is the total number of valence
Q48: Which of the following pure substances has
Q56: What is the K<sub>p</sub> equilibrium-constant expression for
Q63: When cobalt chloride is added to pure
Q67: In a first-order reaction,the half-life is 139
Q85: Assuming the following metals all have the
Q104: The total number of valence electrons in