Consider the following series of molecular ions and molecules: F2+,F22+,F2,and F2-.Which will have the shortest bond length between the fluorine atoms? Assume the homonuclear molecular orbital diagram provided below for nitrogen (excluding the K shells) still applies to these species. 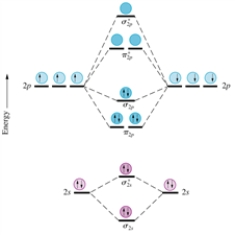
Definitions:
Scores
The quantitative outcomes or measurements that are obtained from tests, assessments, or evaluations.
Two Middle Scores
The median values in a data set, which are the two central numbers in an ordered sequence when the total number of observations is even.
Nominal Level
A measurement level used for categorizing data without a natural order or ranking.
Measurement Precision
The consistency and repeatability of a measurement, not necessarily accurate but consistent.
Q14: Consider the following system at equilibrium: N<sub>2</sub>(g)+
Q18: Which of the following electron configurations corresponds
Q24: The reaction CHCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="The
Q29: What hybrid orbitals of sulfur are involved
Q29: Platinum crystallizes with a face-centered cubic unit
Q34: A 1.67-g sample of solid silver reacted
Q60: Which of the following statements is incorrect?<br>A)One
Q70: All of the following species have ground-state
Q80: The rates of most chemical reactions are
Q82: What is the electron geometry (or electron