Consider the following gas-liquid equilibrium for an aqueous system at a constant partial pressure of N2.
N2(g) 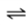
N2(aq)
What is the effect on the equilibrium composition of the liquid when the temperature of the liquid is increased?
Definitions:
Parallelism
The use of components in sentences that are grammatically the same or similar in their construction, sound, meaning, or meter.
Social Media Site
An online platform that enables users to create and share content or participate in social networking with others.
Parallelism
Parallelism refers to the practice of ensuring elements in sentences are grammatically similar or identical in structure, sound, meaning, or meter to enhance coherence and balance.
Bank Debits
Transactions that reduce the account balance of a bank account, representing money that has been withdrawn or payments made.
Q6: Which of the following pure substances has
Q27: A 0.10 M solution of a weak
Q37: The rate constant for a first-order reaction
Q42: A 0.10 M solution of a weak
Q69: Which of the following solutes,dissolved in 1.0
Q71: For which order reaction is the half-life
Q73: In general,atomic radii<br>A)decrease from left to right
Q82: Which of the following expressions is not
Q99: Consider the reaction<br>2HCl(g)→ H<sub>2</sub>(g)+ Cl<sub>2</sub>(g); ΔH =
Q100: Which of the following orbital diagrams represent(s)a