Consider the following gas-liquid equilibrium for an aqueous system at a constant partial pressure of N2.
N2(g) 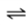
N2(aq)
What is the effect on the equilibrium composition of the liquid when the temperature of the liquid is increased?
Definitions:
Hospital Census
A count of the number of patients in a hospital at a specific time.
General Adaptation Syndrome
A theoretical model describing the body's short-term and long-term reactions to stress, consisting of three stages: alarm, resistance, and exhaustion.
Primary Hormone
A crucial hormone that plays a leading role in regulating vital body processes.
Stress Response
The body's reaction to any change that requires an adjustment or response, involving physical, mental, and emotional responses.
Q1: What is the molecular geometry of the
Q15: The molal boiling-point constant for water is
Q21: What is the equilibrium concentration of H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>
Q23: What is the molecular geometry around an
Q46: The molecular geometry of the ammonium ion,NH<sub>4</sub><sup>+</sup>,is
Q47: What is K<sub>a</sub> for a weak monoprotic
Q52: Which of the following substances,if added to
Q55: In a 0.01 M solution of 1,4-butanedicarboxylic
Q98: Which principle or rule is violated by
Q102: In which of the following lists do