The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the following equation:
OCl-(aq) + I-(aq) → OI-(aq) + Cl-(aq)
The rate law for this reaction is Rate = k 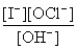
) If time is measured in seconds and concentration is measured in moles per liter,what are the units for k?
Definitions:
General Ledger
The master set of accounts that summarize all transactions occurring within an entity, serving as the main source for financial reporting.
Normal Balance
The side (debit or credit) of an account that is expected to have a higher balance, according to the rules of double-entry accounting.
Debit
An accounting entry that results in either an increase in assets or a decrease in liabilities on a company's balance sheet.
Credit
An arrangement whereby goods, services, or money is received in exchange for a promise to pay back a definite sum of money at a future date.
Q10: What is the minimum concentration of Pb<sup>2+</sup>
Q45: A saturated solution of which of the
Q51: A solution is 0.010 M in each
Q56: What is the hydronium-ion concentration of a
Q69: A sample of ammonia gas was allowed
Q73: Which aqueous solution has the lowest pH?<br>A)0.30
Q75: Which of the following involves a change
Q79: What is the pH of a 0.072
Q85: Assuming the following metals all have the
Q116: Which of the following equilibria best represents