The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the following equation:
OCl-(aq) + I-(aq) → OI-(aq) + Cl-(aq)
The rate law for this reaction is Rate = k 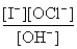
) The overall reaction order and the order with respect to OH- are
Definitions:
PowerPoint Presentations
A series of slides created using Microsoft PowerPoint software, typically used for educational or business presentations to visually communicate information.
Media Literacy
The ability to critically understand and evaluate content across various media formats and platforms.
Magazines
Publications, usually periodical, that contain articles, photographs, and advertisements on various subjects.
Digital Literacy
The ability to find, evaluate, utilize, share, and create content using information technologies and the internet.
Q4: In the reaction<br>CuO(s)+ CO<sub>2</sub>(g)→ CuCO<sub>3</sub>(s),<br>A)O<sup>2-</sup> acts as
Q6: What is the pOH of a 0.0032
Q22: Which molecule or ion has the shortest
Q46: Rank the following ions in order of
Q69: A sample of ammonia gas was allowed
Q71: For which order reaction is the half-life
Q83: The insoluble salts AV,B<sub>2</sub>W,C<sub>2</sub>X<sub>3</sub>,DY<sub>2</sub>,and EZ<sub>3</sub>,which were formed
Q88: What is the mole fraction of toluene
Q89: What is the relationship between molar solubility
Q109: The metal lead,with an atomic radius of