The OH· radical disproportionates according to the elementary chemical reaction 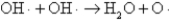 This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
Definitions:
Managerial Behaviour
The patterns of actions and attitudes exhibited by managers, influencing the performance and culture of an organization.
Network Models
Theoretical frameworks that describe how different points or nodes within a network are connected and interact with each other.
Complex Projects
Initiatives characterized by high levels of uncertainty, intricacy, and interdependencies, requiring careful planning and coordination to successfully complete.
Psychological Success
The personal sense of accomplishment and satisfaction attained from achieving professional goals and advancing in one's career.
Q4: What is the hydronium-ion concentration in a
Q6: Which of the following salts is most
Q18: In the ICI<sub>4</sub><sup>-</sup> ion,how many electron groups
Q43: The solubility of a gas in a
Q50: A solution of CF<sub>3</sub>H in H<sub>2</sub>CO is
Q58: Which of the following pure substances has
Q61: A _ is an ion formed from
Q70: What is the concentration of silver(I)ion in
Q72: Given the two equilibria below,<br>Ag(NH<sub>3</sub>)<sub>2</sub><sup>+</sup>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg"
Q110: A suspension of sodium dodecanoate,CH<sub>3</sub>(CH<sub>2</sub>)<sub>10</sub>COONa,in water is