The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the following equation:
OCl-(aq) + I-(aq) → OI-(aq) + Cl-(aq)
The rate law for this reaction is Rate = k 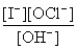
) If time is measured in seconds and concentration is measured in moles per liter,what are the units for k?
Definitions:
Auditing Experience
The professional knowledge and skill gained through performing audits, typically used by accountants and auditors to ensure financial statements are accurate and comply with laws and regulations.
Independence
The state of being free from external control or influence, often related to auditor's neutrality.
Conceptual Framework
A set of fundamental principles and objectives that guides the development of accounting standards and practices.
General Purpose
Aimed to serve a wide range of uses or objectives rather than being specialized for a specific function or task.
Q11: Which of the following statements is true
Q16: Consider the reaction<br>S<sub>2</sub>Cl<sub>2</sub>(l)+ CCl<sub>4</sub>(l) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Consider
Q29: _ states that,when a system in chemical
Q72: Rank the following ions in order of
Q73: Which pure substance exhibits hydrogen bonding?<br>A)HNF<sub>2</sub><br>B)B<sub>2</sub>H<sub>6</sub><br>C)HBr<br>D)H<sub>2</sub>S<br>E)CaH<sub>2</sub>
Q84: The nuclide <sup>188</sup>W decays by a first-order
Q85: If 275 mL of 1 × 10<sup>-7</sup>
Q86: The vapor pressure of a given liquid
Q119: The strongest intermolecular forces between molecules of
Q120: In the Born-Haber cycle for KI(s),which of