The gas-phase decomposition of N2O5 is a first-order process with a rate constant of 1.50 × 10-3 s-1 at 55°C.The decomposition reaction is
N2O5(g) → 2NO2(g) + 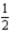
O2(g)
If 8.0 g of N2O5 is placed in vessel 1 and 16.0 g of N2O5 in vessel 2 and the vessels are at the same temperature (55°C) and the same pressure,how much time is required for half of the N2O5 to decompose in each vessel?
Definitions:
Different Behaviors
Varied actions or reactions of individuals in response to external or internal stimuli, differing across contexts or situations.
Unique Segmentation
Segmentation that targets the preferences of a group of consumers with similar needs and wants only within one country.
Similarity
The state or fact of being alike or having common features or attributes.
Psychographics
A marketing research method that analyzes consumer lifestyles, values, attitudes, and personality traits to better understand their buying behavior.
Q3: For which of the following salts would
Q4: For which of the following pairs of
Q13: For which of the following values of
Q27: The rate law for the reaction between
Q68: According to valence-bond theory,the bonding in ketene,H<sub>2</sub>CCO,is
Q88: The following species, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="The following
Q90: What is the pH of a saturated
Q92: Given the accompanying phase diagram,under what conditions
Q106: Which of the following is an ionic
Q109: What is the electron configuration for ?