The OH· radical disproportionates according to the elementary chemical reaction 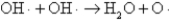 This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
Definitions:
Withdrawals
Money taken out from a bank account or the act of removing assets from a business by the owner for personal use.
Premium for Admission
An additional charge over the regular price for entry into an organization, service, or area typically offering greater benefits.
Bonus
A bonus is a form of additional compensation paid to an employee above their normal wage, often as a reward for reaching specific performance targets or for exceptional work.
Capital Accounts
Accounts on a company's balance sheet representing the original investment plus retained earnings and adjustments.
Q38: For a solution containing only one solute
Q53: Which of the following is the strongest
Q66: In a(n)_ solution,at 25°C,the concentrations of H<sub>3</sub>O<sup>+</sup>
Q81: Which of the following characteristics does not
Q82: What is the base-ionization equilibrium constant for
Q97: Which of the following salts is most
Q98: Which of the following statements is incorrect?<br>A)The
Q101: Which of the following indicates the existence
Q106: Which of the following is an ionic
Q134: Which of the following indicators is most