A proposed mechanism for the decomposition of N2O5 is as follows:
N2O5 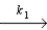
NO2 + NO3
Slow step
NO2 + NO3 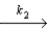
NO2 + O2 + NO
Fast step
NO + N2O5 
3NO2
Fast step
What is the overall chemical equation predicted by this mechanism?
Definitions:
Economies Of Scope
Cost advantages that a business experiences by producing a variety of products rather than specializing in a single product or service.
Economies Of Scope
Cost advantages that a business obtains through a variety of products rather than specializing in a single product.
Synergy
The concept that the value and performance of two companies combined will be greater than the sum of the separate individual parts.
Cost Complementarity
Occurs when the cost of producing one good decreases with the increase in production of another good, showing a synergy between the production processes of two goods.
Q59: What is the K<sub>c</sub> equilibrium-constant expression for
Q61: What is the percent ionization of a
Q61: The electronic structure of which of the
Q62: The reaction 3NO → N<sub>2</sub>O + NO<sub>2</sub>
Q68: A cucumber is placed in a concentrated
Q71: Which molecule or ion is nonlinear?<br>A)N<sub>2</sub>O<br>B)O<sub>3</sub><br>C)OCN<sup>-</sup><br>D)NO<sub>2</sub><sup>+</sup><br>E)CS<sub>2</sub>
Q77: Which of the following pure substances has
Q106: Which of the following salts has the
Q113: What is the solubility product expression for
Q123: A 0.20 M solution of a weak