When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilbrium mixture of reactants and products according to the balanced chemical equilibrium below.
CO(g) + 3H2(g) 
CH4(g) + H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve B? 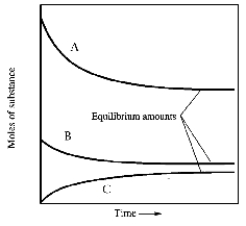
Definitions:
Marketing Metrics
Quantitative measures used to track the performance of marketing activities and campaigns.
Store Visit
The act of potential customers or consumers physically visiting a retail or merchandise location to purchase or inspect products.
Markdown Percentage
The reduction in the selling price of goods, expressed as a percentage of the original price, usually to clear inventory or stimulate sales.
Average Transaction Size
The average amount spent by customers in a single transaction or purchase.
Q14: Which of the following species cannot act
Q43: Which molecule or ion is not planar?<br>A)XeF<sub>4</sub><br>B)NO<sub>3</sub><sup>-</sup><br>C)BCl<sub>3</sub><br>D)F<sub>2</sub>CCF<sub>2</sub><br>E)CF<sub>4</sub>
Q46: Rank the following in order of decreasing
Q51: Which of the following is the strongest
Q75: A sample of ammonia gas was allowed
Q85: What is the equilibrium concentration of ammonium
Q92: For which of the following systems at
Q99: When an atom in a molecule or
Q100: The hypochlorite ion oxidizes the iodide ion
Q110: What is the hydrogen-ion concentration of a