Given the equilibrium constants for the equilibria,
22NH4+(aq) + 2H2O(l) 
2NH3(aq) + 2H3O+(aq) ; Kc = 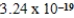
CH3COOH(aq) + H2O(l) 
CH3COO-(aq) + H3O+(aq) ; Kc = 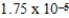
Determine Kc for the following equilibrium.
CH3COOH(aq) + NH3(aq) 
CH3COO-(aq) + NH4+(aq)
Definitions:
Spike Suppression
Techniques or devices used to protect electronic circuits from sudden transient voltages, which could cause damage or malfunction.
Metal Oxide Varistor
A voltage-dependent resistor with a nonlinear variance, used to protect electrical circuits from voltage spikes.
Inductance
The property of an electrical conductor by which a change in current flowing through it induces an electromotive force in both the conductor itself and in any nearby conductors by mutual inductance.
Wire Resistance
The electrical resistance of a wire, determined by its material, length, and cross-sectional area, affecting the flow of electric current through it.
Q3: For which of the following salts would
Q11: Which of the following species cannot act
Q25: For a reaction that has an equilibrium
Q25: Which of the following statements is true
Q53: A solution of two liquids,A and B,shows
Q66: The metal niobium crystallizes in a body-centered
Q68: The concentration of barium carbonate in a
Q73: The configuration (σ<sub>2s</sub>)<sup>2</sup>(σ<sub>2s</sub>*)<sup>2</sup>(π<sub>2py</sub>)<sup>1</sup>(π<sub>2px</sub>)<sup>1</sup> is the molecular orbital
Q78: Lithium chloride crystallizes in a face-centered cubic
Q103: The solubility of La(IO<sub>3</sub>)<sub>3</sub> in a 0.71