An initially 1.0 M aqueous solution of a weak monoprotic acid has a total ion concentration of 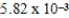 M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)
M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)
Definitions:
Breach Of Contract
Not meeting any condition of a contract, be it in written or oral form, without an authentic legal reason.
Creditor Beneficiary
A third party who benefits from a contract in which the promisor agrees to pay the promisee’s debt.
Donee
The recipient of a gift or donation, who is typically not required to provide anything in return to the donor.
Incidental
Occurring as a minor accompaniment to something else or happening as a subordinate or secondary aspect.
Q18: What is the hydronium-ion concentration at equilibrium
Q28: Which of the following acids has the
Q33: A solution has a pH of 10.20
Q53: Which of the following is the strongest
Q55: A second-order reaction starts with an initial
Q60: The balanced chemical equation and rate law
Q61: The rate law for the chemical reaction<br>5Br<sup>-</sup>(aq)+
Q69: Consider the following reaction,which is spontaneous at
Q72: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q108: Which reaction would be most likely to