The amphiprotic anion monohydrogenphosphate acts as both an acid and a base in aqueous solution.Given the following acid-ionization and base-hydrolysis constants,what will be the approximate equilibrium pH of an aqueous solution of sodium monohydrogenphosphate?
Ka = 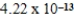
Kb = 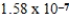
Definitions:
Sample Mean
The average of a sample set of numbers, calculated by adding up all the numbers and dividing by the count of numbers in the set.
Sample Mean
The average value of a sample set of numbers.
Freshmen
First-year students in a secondary or post-secondary educational institution.
Probability
The evaluation of how probable an event is, shown as a numerical value between 0 and 1, where 0 stands for the event being impossible and 1 representing absolute certainty of the event occurring.
Q2: Calculate the pH of a solution that
Q7: _ is a thermodynamic quantity that is
Q13: A living tree contains <sup>14</sup>C (half-life,5730 years)and
Q15: The ionization constant of water at a
Q26: What is the percent ionization of a
Q28: For the process Cl<sub>2</sub>(g)→ 2Cl(g),<br>A)ΔH is +
Q43: What is the pOH of a solution
Q69: The K<sub>a</sub> for hydrofluoric acid is 6.8
Q70: For the following reaction system at equilibrium,which
Q95: Balance the following oxidation-reduction occurring in acidic