Multiple Choice
The equilibrium hydronium ion concentration of an initially 0.510 M solution of a diprotic weak acid (H2A) is 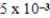 M.What is Ka1? (assume Ca/Ka ≥ 102 and Ka1 >> Ka2)
M.What is Ka1? (assume Ca/Ka ≥ 102 and Ka1 >> Ka2)
Definitions:
Related Questions
Q10: In Arrhenius theory,a(n)_ is a substance that
Q13: What is the concentration of Cd<sup>2+</sup> in
Q22: Which solution has the highest pH?<br>A)0.1 M
Q27: The solubility of calcium carbonate in water
Q43: What is the pOH of a solution
Q47: Which of the following is not a
Q83: What is the pH of a buffer
Q91: For the reaction A + B +
Q98: In the titration of a weak monoprotic
Q134: Which of the following indicators is most