The amphiprotic anion monohydrogenphosphate acts as both an acid and a base in aqueous solution.Given the following acid-ionization and base-hydrolysis constants,what will be the approximate equilibrium pH of an aqueous solution of sodium monohydrogenphosphate?
Ka = 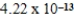
Kb = 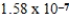
Definitions:
Luxury Chocolates
High-quality chocolates that are often handcrafted with premium ingredients and unique flavors, positioned as gourmet or upscale products.
Brand Awareness
The level of awareness customers have about a specific brand's attributes or reputation.
Top-Of-Mind Awareness
The initial product or brand a consumer recalls when considering a specific category or industry.
Specific Brand
A unique design, symbol, or name that identifies and distinguishes a product or service from others in the market.
Q12: What is the balanced equation for the
Q27: The rate law for the reaction between
Q30: Which of the following statements is true
Q49: What is the percent activity of a
Q54: What is ΔG° at 500.0 K for
Q61: Relative to the sum of the masses
Q78: What is the mole fraction of water
Q92: According to the first law of thermodynamics,the
Q93: According to the National Institute of Standards
Q95: Which of the following statements is incorrect?<br>A)The