Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.21 mol of Zn(NH3) 42+ per liter and 0.4986 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 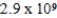 .
.
Definitions:
Deindividuation
A psychological state of decreased self-awareness and decreased social identity, leading to uncharacteristic, often disinhibited behavior.
Diverse Groups
Collections of individuals with varying characteristics, backgrounds, experiences, and perspectives.
Similarity
The state of being alike or sharing common features, characteristics, or attributes.
Team Members
Individuals who are part of a group collaborating towards a common goal or project.
Q23: What is the equilibrium pH of an
Q35: For which of the following processes would
Q35: A lead storage battery involves the following
Q38: Determine the molecularity of the following elementary
Q47: Which of the following is not a
Q49: What is the metallurgical process of roasting?<br>A)A
Q69: For the cell Cu(s)|Cu<sup>2+</sup>||Ag<sup>+</sup>|Ag(s),the standard cell potential
Q76: Solid KCN is added to a solution
Q78: Calculate the solubility product of silver iodide
Q109: What is the hydronium-ion concentration of a