What is the molar equilibrium concentration of uncomplexed Fe2+(aq) in a solution composed of 1.4 mol Fe(CN) 64− dissolved in 1.00 L of 0.33 M NaCN.Kf for Fe(CN) 64− is 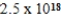 .
.
Definitions:
High Status
A position of authority, respect, or prestige in a social hierarchy or organization.
Pleasure
A feeling of happiness, enjoyment, or satisfaction derived from experiences that are enjoyable or gratifying.
Withdrawal Distress
The physical and emotional symptoms experienced after discontinuing the use of an addictive substance.
Heroin Addicts
Individuals who suffer from addiction to heroin, a highly addictive opioid drug, often facing significant physical, psychological, and social consequences.
Q6: What is the pOH of a 0.0032
Q28: For the process Cl<sub>2</sub>(g)→ 2Cl(g),<br>A)ΔH is +
Q42: Which alkali metal is capable of forming
Q43: A particular nuclear bombardment reaction is represented
Q54: How many faradays are required to convert
Q61: A _ is an ion formed from
Q86: Which of the following statements is true
Q87: In order to determine the identity of
Q118: In which of the following compounds does
Q132: For which of the following equilibria does