Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.21 mol of Zn(NH3) 42+ per liter and 0.4986 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 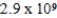 .
.
Definitions:
Annual Net Cash Inflows
The total amount of cash received minus cash expenses over a year.
Net Present Value
The discrepancy in the present valuation of incoming and outgoing cash flows within a certain timeframe.
Useful Life
The estimated duration of time a fixed asset is considered to be productive for its intended use.
Working Capital
is the difference between a company's current assets and current liabilities, indicating the short-term financial health and operational efficiency.
Q12: Which of the following solutions would show
Q16: The reaction between selenous acid and the
Q23: The formula for a platinum(IV)complex is [Pt(NH<sub>3</sub>)<sub>2</sub>Br<sub>2</sub>]Cl<sub>2</sub>.In
Q24: The reaction CHCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="The
Q27: What is the formula for the hexaaquavanadium(II)ion?<br>A)[V(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup><br>B)[V(H<sub>2</sub>O)<sub>4</sub>]<sup>2+</sup><br>C)[V<sub>2</sub>(H<sub>2</sub>O)<sub>6</sub>]<sup>4-</sup><br>D)[V<sub>2</sub>(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup><br>E)[V<sub>2</sub>(H<sub>2</sub>O)<sub>6</sub>]<sup>4+</sup>
Q33: Which of the following metals tends to
Q51: Below is a proposed mechanism for the
Q54: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q60: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="For
Q105: The two acid-ionization constants for sulfurous acid,H<sub>2</sub>SO<sub>3</sub>,are