What is the molar equilibrium concentration of uncomplexed Fe2+(aq) in a solution composed of 1.4 mol Fe(CN) 64− dissolved in 1.00 L of 0.33 M NaCN.Kf for Fe(CN) 64− is 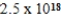 .
.
Definitions:
Estimated Life
The expected period during which an asset is considered to be useful in operations, affecting depreciation calculations.
Capital Expenditure
Resources deployed by a business to buy, enhance, and manage material assets, including real estate, manufacturing facilities, or devices.
Debit
Amount entered on the left side of an account.
Expense Account
An accounting entry that represents consumption of assets, decrease in owners' equity, or incurrence of liabilities during a period as a result of operational activities.
Q29: In which of the following ions does
Q30: When the radioactive nuclide <sup>110</sup>In undergoes positron
Q34: What is the molar solubility of MgF<sub>2</sub>
Q38: Which of the following is the best
Q42: One mechanism for the depletion of ozone
Q43: Consider the following equilibrium.<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg"
Q65: When the following oxidation-reduction reaction in acidic
Q71: A solution contains 0.08 M acetic acid,HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>
Q75: Which of the following compounds is not
Q92: Cyanide ion forms very stable complex ions