Given the following equilibrium constants,
Zn(IO3) 2 Ksp = 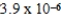
Zn(NH3) 42+ Kf = 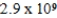
Determine Kc for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
Zn(IO3) 2(s) + 4NH3(aq)  Zn(NH3) 42+(aq) + 2IO3-(aq)
Zn(NH3) 42+(aq) + 2IO3-(aq)
Definitions:
Largest Polluter
The entity or country that contributes the most to environmental degradation through the release of harmful substances and pollutants.
Nations
Large groups of people who share common historical, cultural, or linguistic roots, inhabiting a particular country or territory.
Contamination
The presence or introduction of harmful or undesirable substances, making something impure or unfit for use.
Indigenous Peoples
Ethnic groups who are the original inhabitants of a region, and maintain traditions distinct from the dominant culture of a country.
Q7: A radioactive isotope decays by α emission
Q11: Identify the rate equation of the
Q15: All of the following elements have the
Q47: A radioactive isotope undergoes decay by emission
Q51: What is K<sub>a</sub> at 25°C for the
Q54: Consider the following equilibrium:<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg"
Q61: When Au is obtained by electrolysis from
Q71: For a particular process,q = 20 kJ
Q93: The following equilibrium is exothermic.How could the
Q119: Which of the following equilibria best represents