What is the balanced spontaneous reaction and standard cell potential of an electrochemical cell constructed from half cells with the following half reactions?
Cu2+(aq) + 2e¯ → Cu(s) E° = 0.337 V 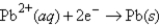
E° = -0.130 V
Definitions:
Tricarboxylic Acid Cycle
A series of chemical reactions used in aerobic respiration to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins.
Oxaloacetate
A 4-carbon compound; an important intermediate in the citric acid cycle and in the.
Acetyl CoA
A molecule that plays key roles in metabolism, participating in synthesis and oxidation processes within cells.
Anaerobic Respiration
A form of respiration that occurs in the absence of oxygen, producing energy and by-products like lactic acid or ethanol.
Q13: Assuming ΔH and ΔS are constant with
Q21: What is the molar solubility of MgF<sub>2</sub>
Q39: Which of the following pairs of substances
Q47: Which of the following will apply to
Q49: What is the metallurgical process of roasting?<br>A)A
Q52: What is the solubility product expression for
Q68: What is the cell reaction for the
Q74: -Given the following,determine ΔG° at 298 K
Q75: Which is the correct condensed structural formula
Q92: According to the first law of thermodynamics,the