A voltaic cell is made by placing an iron electrode in a compartment in which the Fe2+ concentration is 2.0 × 10-5 M and by placing a Pt electrode in the other compartment,in which the H+ concentration is 3.4 M and 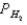 = 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is the value of E° for this cell,and which electrode is the anode?
= 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is the value of E° for this cell,and which electrode is the anode?
Definitions:
Income Distribution
The way in which total income is shared among the population or different groups within society.
Environmental Scanning
The process of systematically gathering information about the external environment, including trends and changes, to aid in strategic planning.
Global Marketing Environment
The external factors in international markets that affect marketing strategies, including economic, cultural, legal, and technological factors.
Global Scale
Refers to the extent or level at which a process, activity, or phenomenon is studied or applied across the entire world.
Q3: Which of the following reactions does not
Q30: A 0.010 M aqueous solution of a
Q32: From these two reactions at 298 K,<br>V<sub>2</sub>O<sub>3</sub>(s)+
Q59: Molecules that have nonsuperimposable mirror images are<br>A)multidentate.<br>B)racemic.<br>C)dextrorotatory.<br>D)chiral.<br>E)levorotatory.
Q64: Which of the following particles cannot be
Q66: The functional group <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="The functional
Q70: For a certain oxidation-reduction reaction,E°<sub>cell</sub> is negative.This
Q72: What is the major product upon addition
Q73: Silver nitrate (AgNO<sub>3</sub>)is slowly added to a
Q91: ΔH and ΔU are nearly the same