A voltaic cell is made by placing an iron electrode in a compartment in which the Fe2+ concentration is 2.0 × 10-5 M and by placing a Pt electrode in the other compartment,in which the H+ concentration is 3.4 M and 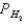 = 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at equilibrium?
= 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at equilibrium?
Definitions:
Loaded
Often relates to questions or statements designed to elicit emotional responses or contain an implicit assumption.
Yea-Saying
The tendency of respondents to agree with statements or questions regardless of their actual opinion, often leading to bias in survey responses.
Loaded
A term used to describe questions or statements that are designed to elicit emotional responses or include assumptions, potentially biasing the response.
Simplistic
An approach or explanation that oversimplifies a concept or issue, often ignoring complexities and nuances.
Q10: Which of the following nuclides will produce
Q22: Which of the following statements is true
Q28: What is the correct IUPAC name for
Q30: Which of the following statements is true
Q64: What is the solubility product expression for
Q71: For a particular process,q = 20 kJ
Q79: The gamma ray emission from the decay
Q86: Which of the following statements is true
Q102: What is the pH of a solution
Q143: For a 0.05 M H<sub>2</sub>SO<sub>3</sub> solution,which of