What is the value of the reaction quotient,Q,for the voltaic cell constructed from the following two half-reactions when the Zn2+ concentration is 0.0120 M and the Ag+ concentration is 1.27 M? 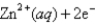

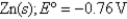
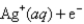

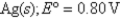
Definitions:
Q7: How many unpaired electrons are there in
Q35: For which of the following processes would
Q42: The sequence of amino acids held together
Q51: Given:<br>Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q54: What is the polymer formed by the
Q64: Which of the following reactions has the
Q71: When an aqueous solution of AgNO<sub>3</sub> is
Q71: The hydronium-ion concentration of a solution is
Q78: The standard free energy of formation of
Q105: Molten magnesium chloride is electrolyzed using inert