A solution of 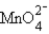 is electrolytically reduced to Mn3+.A current of 5.19 A is passed through the solution for 15.0 min.What is the number of moles of Mn3+ produced in this process? (1 faraday = 96,486 coulombs)
is electrolytically reduced to Mn3+.A current of 5.19 A is passed through the solution for 15.0 min.What is the number of moles of Mn3+ produced in this process? (1 faraday = 96,486 coulombs)
Definitions:
Equity Index
A benchmark that measures the performance of a specific section of the stock market by tracking the price of selected stocks.
Volatility
A statistical measure of the dispersion of returns for a given security or market index, often used as a measure of risk.
U.S. Dollar-Denominated Returns
Investment returns calculated in U.S. dollars, highlighting the effect of currency fluctuation on international investments for U.S. investors.
Lowest Volatility
The condition of experiencing the smallest degree of variation in price over a specified period, often sought after in stable investment options.
Q3: Which of the following reactions does not
Q6: Which of the following is not an
Q32: Which of the following combinations is not
Q45: A saturated solution of which of the
Q54: What is the polymer formed by the
Q74: 15.0 mL of 0.50 M HCl is
Q76: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q78: Metastable isotopes,such as Technetium-99m and those produced
Q85: Catenation is<br>A)the ability of a compound to
Q104: You have two salts,AgX and AgY,with very