What is the balanced spontaneous reaction and standard cell potential of an electrochemical cell constructed from half cells with the following half reactions?
Cu2+(aq) + 2e¯ → Cu(s) E° = 0.337 V 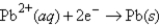
E° = -0.130 V
Definitions:
Dividend Payout Ratio
A financial metric that calculates the percentage of net income distributed to shareholders in the form of dividends.
Total Stockholders' Equity
The total net worth of a company according to its balance sheet, calculated as total assets minus total liabilities.
Common Stock
A type of equity security representing ownership in a corporation, with holders typically having voting rights and receiving dividends.
Dividend Yield Ratio
A financial ratio that indicates how much a company pays out in dividends each year relative to its stock price.
Q18: A solution has a hydronium-ion concentration of
Q24: Enriched uranium is uranium that has a
Q36: What is ΔG° at 298 K for
Q50: What is the nitrogenous base that is
Q60: Iodine-131 decays by beta emission with a
Q66: Which of the following is not a
Q68: What is the change in entropy when
Q76: For a reaction system that is at
Q77: Which of the following mixtures will be
Q94: What is the solubility (in g/L)of calcium