A voltaic cell is made by placing an iron electrode in a compartment in which the Fe2+ concentration is 2.0 × 10-5 M and by placing a Pt electrode in the other compartment,in which the H+ concentration is 3.4 M and 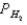 = 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is the value of E° for this cell,and which electrode is the anode?
= 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is the value of E° for this cell,and which electrode is the anode?
Definitions:
Deployment
The action of moving military forces or resources into position for strategic or operational purposes.
Police Use Of Excessive Force
Refers to situations where law enforcement officers apply more physical force than is necessary or justified in the context of an arrest, crowd control, or other policing actions, raising concerns about abuse of power and human rights.
Institutional Culture
The shared values, beliefs, and practices that shape the behavior and structure of an organization or institution.
Societal Pressures
The force exerted by society on individuals to conform to expected norms and behaviors.
Q1: In complexes of platinum(IV),the coordination number is
Q11: The nucleic acid sequence that is complementary
Q24: Which of the following statements concerning aluminum
Q37: For a reversible phase change at constant
Q57: For a certain process,at 300.K,ΔG = -37.2
Q72: Given the two equilibria below,<br>Ag(NH<sub>3</sub>)<sub>2</sub><sup>+</sup>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg"
Q89: What is the relationship between molar solubility
Q102: The best explanation for the dissolution of
Q103: The solubility of La(IO<sub>3</sub>)<sub>3</sub> in a 0.71
Q122: For which of the following equilibria does