A voltaic cell is made by placing an iron electrode in a compartment in which the Fe2+ concentration is 2.0 × 10-5 M and by placing a Pt electrode in the other compartment,in which the H+ concentration is 3.4 M and 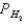 = 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at equilibrium?
= 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at equilibrium?
Definitions:
Normally Distributed
Describes a distribution that follows a normal curve or bell-shaped pattern, where most of the data points are close to the mean.
Standard Deviation
Standard Deviation is a measure of the amount of variability or dispersion around the mean value of a set of data.
Hypothesized Mean
Refers to a specific value of the mean in the population that is proposed for testing in hypothesis testing.
Standard Deviation
A measure that indicates the amount of variation or dispersion of a set of values.
Q4: Which of the following salts would be
Q38: What is the pH of a solution
Q47: Which of the following will apply to
Q48: Which of the following is not a
Q51: Given:<br>Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q54: How many different isomers are possible for
Q69: For the cell Cu(s)|Cu<sup>2+</sup>||Ag<sup>+</sup>|Ag(s),the standard cell potential
Q69: Which of the following properties of the
Q71: Which of the following nuclides will produce
Q144: What is the equilibrium percent ionization of