What is the value of the reaction quotient,Q,for the voltaic cell constructed from the following two half-reactions when the Zn2+ concentration is 0.0120 M and the Ag+ concentration is 1.27 M? 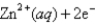

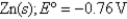
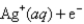

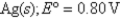
Definitions:
Activity-Based Costing
A costing method that assigns costs to products or services based on the resources they consume, aiming for more accurate costing and insights.
Preparing Deliveries
The process involved in getting goods ready for shipment to customers.
Time-Driven
pertains to processes or activities that are scheduled or executed based on specific time requirements.
Activity-Based Costing
A pricing approach that allocates overhead and indirect expenses to distinct activities, resulting in more precise costing for products or services.
Q3: What is K<sub>a</sub> for the anilinium cation,C<sub>6</sub>H<sub>5</sub>NH<sub>3</sub><sup>+</sup>,at
Q10: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5063/.jpg" alt=" _ Embryoblast A)A
Q17: Which of the following is the most
Q19: What is the principal source of commercial
Q25: For a reaction that has an equilibrium
Q49: Which of the following is not an
Q63: The following reaction represents a step in
Q69: Four of the following compounds are structural
Q91: ΔH and ΔU are nearly the same
Q141: Which of the following statements is true