A voltaic cell is made by placing an iron electrode in a compartment in which the Fe2+ concentration is 3.0 × 10-5 M and by placing a Pt electrode in the other compartment,in which the H+ concentration is 3.10 M and 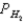 = 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at 25oC?
= 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at 25oC?
Definitions:
Economic Systems
The means by which countries or communities organize and distribute available resources, goods, and services across the economy.
North Korea
North Korea is a country in East Asia, officially known as the Democratic People's Republic of Korea, known for its authoritarian government and isolation from most of the international community.
South Korea
A country located in East Asia, known for its advanced technology sector, vibrant culture, and strong economy.
Corporation
A legal entity recognized under law as separate from its owners, providing limited liability and allowing ownership through shareholding.
Q13: What is the pH of a 0.35
Q16: How many unpaired electrons are there in
Q32: From these two reactions at 298 K,<br>V<sub>2</sub>O<sub>3</sub>(s)+
Q34: Which statement concerning the anode in an
Q56: What is the hydronium-ion concentration of a
Q70: What is the concentration of silver(I)ion in
Q74: -Given the following,determine ΔG° at 298 K
Q104: If an electrolysis plant operates its electrolytic
Q104: You have two salts,AgX and AgY,with very
Q136: What is the pOH of a solution