The major industrial source of hydrogen gas is the reaction of methane and water at high temperatures (800-1000°C) and high pressures (10-15 atm) with nickel as a catalyst. 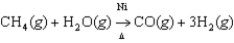 If 150.0 g of CH4 and 150.0 g of H2O are reacted at 915°C and 11.0 atm,how much hydrogen should be available for industrial use?
If 150.0 g of CH4 and 150.0 g of H2O are reacted at 915°C and 11.0 atm,how much hydrogen should be available for industrial use?
Definitions:
Support Product
Ancillary products or services that complement the main product or service offering, enhancing its value or utility.
Industrial Services
Services provided to businesses or industries, such as maintenance, cleaning, consulting, and logistics, rather than individual consumers.
Complementary Products
Goods or services that enhance or are used together with another product, increasing its value or utility.
Stock Keeping Unit
A scannable barcode, often seen as a number or code on products, that allows items to be tracked in inventory.
Q2: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5063/.jpg" alt=" _ Gives rise
Q6: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5063/.jpg" alt=" _ Derived from
Q17: What is the IUPAC name for the
Q21: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5063/.jpg" alt=" _ Decidua parietalis
Q34: Which of the following statements is true
Q41: A strip of iron is placed in
Q53: What is the oxidation state of iron
Q89: For which of the following radioactive decay
Q92: According to the first law of thermodynamics,the
Q105: Molten magnesium chloride is electrolyzed using inert