The major industrial source of hydrogen gas is the reaction of methane and water at high temperatures (800-1000°C) and high pressures (10-15 atm) with nickel as a catalyst. 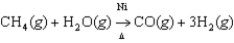 If 150.0 g of CH4 and 150.0 g of H2O are reacted at 915°C and 11.0 atm,how much hydrogen should be available for industrial use?
If 150.0 g of CH4 and 150.0 g of H2O are reacted at 915°C and 11.0 atm,how much hydrogen should be available for industrial use?
Definitions:
Employee Referrals
A recruitment method where existing employees recommend potential candidates for vacancies within the organization, often incentivized by referral programs.
Employee Turnover
The rate at which employees leave a company and are replaced by new hires over a given period, often viewed as an indicator of workplace satisfaction and stability.
University
An institution of higher education and research which awards academic degrees in various academic disciplines.
Job Requirements
The specific qualifications, skills, experience, and characteristics needed to perform a job effectively.
Q18: The _ has zinc can as the
Q22: Nitric acid is obtained commercially from<br>A)NH<sub>3</sub>.<br>B)NH<sub>4</sub>NO<sub>3</sub>.<br>C)Mg<sub>3</sub>N<sub>2</sub>.<br>D)NaNO<sub>2</sub>.<br>E)CO(NH<sub>2</sub>)<sub>2</sub>.
Q51: What is the change in entropy when
Q53: What is the oxidation state of iron
Q53: What is the molar solubility of silver(I)chloride
Q58: What is the major product after addition
Q69: Which of the following properties of the
Q88: Which Figures I-IV represent(s)the result of mixing
Q106: Consider the following cell reaction:<br>2Cr(s)+ 6H<sup>+</sup>(aq)→ 2Cr<sup>3+</sup>(aq)+
Q115: Which of the following elements is obtained