What mass of oxygen gas would be consumed by the complete combustion of 7.5 g of a mixture of propane (C3H8) and butane (C4H10) in the mole ratio of 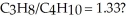
Definitions:
Securities and Exchange Commission
A U.S. federal agency responsible for regulating the securities industry, including enforcing federal securities laws and regulating securities exchanges.
Executive Compensation
The financial and non-financial rewards given to senior management and executives of a company, which may include salary, bonuses, stock options, and other benefits.
Company's Performance
The overall effectiveness and success of a company in achieving its objectives, often measured through financial, operational, and customer satisfaction metrics.
Organizational Performance
Refers to how well an organization is doing in terms of reaching its objectives, which can include financial results, customer satisfaction, and operational efficiency.
Q10: Determine the number of cm<sup>2</sup> in one
Q25: Choose the INCORRECT name formula combination.<br>A)Fe<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub> iron(III)sulfate<br>B)HBrO<sub>3</sub>
Q33: How many grams of N<sub>2</sub> are required
Q60: The heat of combustion of methane is
Q64: Balance the following equation for an oxidation-reduction
Q74: Because of the high heat and low
Q76: You have three cylinders containing O<sub>2</sub> gas
Q88: The composition refers to the components of
Q88: 14.0 g of metal at 24.0 °C
Q118: Two hundred mL of solution has a