Identify the coefficient of NO(g) when the following reaction is balanced,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 12.0 g of oxygen at 25 °C.The density of nitrogen monoxide at 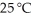
Is 1.23 g L-1.
(unbalanced) NH3(g) + O2(g) → NO(g) + H2O(l)
Definitions:
Cones
Photoreceptor cells in the retina of the eye that are responsible for color vision and function best in relatively bright light.
Non-rapid Eye Movement Sleep
A type of sleep characterized by slower brain waves, limited eye movement, and reduced muscle activity, occupying most of the sleep cycle and associated with deep restorative sleep.
Fetal Hormones
Hormones produced by the fetus during pregnancy that play crucial roles in its development, including the differentiation of sex and the maturation of bodily systems.
Anesthetics
Drugs used during surgical procedures to induce a loss of sensation or consciousness, allowing for pain-free surgeries.
Q33: Use the basic rules for electron configuration
Q37: Round off 00907506 to four significant figures.<br>A)0091<br>B)9076<br>C)9100<br>D)9.075
Q40: Choose the INCORRECT statement.<br>A)The surroundings are the
Q51: How many atoms of sulfur are in
Q86: Enthalpy is an extensive property.
Q93: Calculate the work needed to make room
Q94: To measure the volume of an irregularly
Q97: White phosphorus,P<sub>4</sub>,spontaneously bursts into flame in oxygen.If
Q107: A sample of oxygen gas is collected
Q131: Combustion analysis of a 22.0 mg sample