What mass of trisodium phosphate is required to prepare 250.0 mL of a solution that is 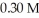
In sodium ion?
Definitions:
Market Transaction
An exchange of goods, services, or financial assets in return for money between parties within a marketplace.
Emission Control Devices
Technologies or equipment installed on vehicles or industrial plants to reduce pollutants released into the atmosphere.
External Costs
Costs that are not borne by the parties involved in an economic transaction but by other individuals or society at large.
Q11: Silver possesses two stable isotopes: <sup>107</sup>Ag (106.90
Q23: If the density of lead is 11.34
Q38: Which statement is INCORRECT?<br>A)Natural laws are concise
Q48: What is the correct answer,with the proper
Q55: A solution is prepared by mixing 50.0
Q69: Which of the following is a characteristic
Q80: Calculate the concentration (M)of sodium ions in
Q88: Lead-206 has a relative abundance of 23.6%.What
Q103: How many moles of Co<sup>2+</sup> (aq)are present
Q105: What reagent could not be used to