Identify the coefficient of NO(g) when the following reaction is balanced,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 12.0 g of oxygen at 25 °C.The density of nitrogen monoxide at 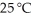
Is 1.23 g L-1.
(unbalanced) NH3(g) + O2(g) → NO(g) + H2O(l)
Definitions:
Photosynthetic Prokaryotes
Microorganisms that possess the ability to carry out photosynthesis, such as cyanobacteria, without a defined nucleus.
Primitive Atmosphere
Describes the early Earth's atmosphere, which lacked oxygen and was composed mainly of carbon dioxide, water vapor, nitrogen, and inert gases.
Methane
A potent greenhouse gas with a significant impact on global warming, primarily produced from decomposition of organic matter and the digestive processes of certain animals.
Carbon Dioxide
A gas that has no color or smell, created when carbon and organic compounds burn and during breathing. It exists naturally in the atmosphere and is taken in by plants during the process of photosynthesis.
Q18: Moseley used X-rays to determine the atomic
Q19: Which ion does not have a noble
Q44: A sample contains 43.7% phosphorus and 56.3%
Q50: Robert Millikan determined the charge on an
Q60: The average atomic mass of B is
Q83: Calculate the percent abundance of the two
Q90: The possible values of the magnetic quantum
Q107: The formula of barium nitride is _.<br>A)Ba<sub>3</sub>N<sub>2</sub><br>B)Ba<sub>2</sub>N<sub>2</sub><br>C)BaN<br>D)Ba<sub>2</sub>N
Q112: The volume of 350.mL of gas at
Q143: What type of bonding is found in