Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: 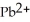
(aq) + 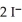
(aq) → 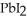
(s)
Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many millilitres of 3.550 mol L-1 HI(aq) must be added to a solution containing 0.700 mol of 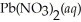
To completely precipitate the lead?
Definitions:
Incompatible Needs
Situations or desires that cannot be satisfied or met simultaneously due to conflicting requirements or circumstances.
Performance Goals
Targets or objectives set to achieve specific performance standards, often characterized by a focus on improving or maintaining the quality of results.
Perfection
The state of being completely free from faults or defects, or the highest degree of proficiency in something.
Approach
The method or process taken towards dealing with a problem or task.
Q11: Calcium oxide reacts with water in a
Q14: The complete electron configuration of nickel,element 28,is:<br>A)
Q17: The effective nuclear charge for sodium is:<br>A)+10<br>B)+9<br>C)+1<br>D)+11<br>E)0
Q24: For the Bohr hydrogen atom determine the
Q34: Three volumes of O<sub>2</sub> and one volume
Q42: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="For the
Q79: Complete the equation and indicate if a
Q91: What concentration of Fe<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>(aq)is required for the
Q112: Which of the following is most likely
Q113: How many grams of calcium chloride are