In the reaction 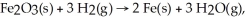
How many moles of iron can be produced using 17.4 liters of hydrogen at STP?
Definitions:
Carboxylic Acid
An organic compound containing a carboxyl group (C(O)OH), known for its acidity due to the hydrogen atom's ability to dissociate from the carboxyl group.
Acid Chloride
Organic compounds derived from carboxylic acids by replacing the hydroxyl group with a chlorine atom, making them highly reactive acylating agents.
Anhydride
A compound formed from another compound by the removal of a molecule of water; commonly associated with carboxylic acid anhydrides in organic chemistry.
Nitrile
Organic compounds that contain a cyano group (-C≡N) attached to an alkyl group, used in the manufacture of rubbers, resins, and pharmaceuticals.
Q7: Identify the reducing agent in the following
Q14: Given the bond enthalpies C-C (348),C=O (707),O
Q44: A sample contains 43.7% phosphorus and 56.3%
Q64: Which series below represents the correct order
Q82: The electron affinity is the energy for
Q93: Arrange the following in order of increasing
Q103: How many moles of Co<sup>2+</sup> (aq)are present
Q112: What are the possible values of l
Q116: The oxidation state of vanadium in VO<sup>2+</sup>
Q123: The substance C<sub>5</sub>H<sub>5</sub>OH in water solution is