The complete combustion of octane,a component of gasoline,is represented by the equation:
2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(l)
How many liters of CO2(g) ,measured at 63.1 °C and 688 mmHg,are produced for every gallon of octane burned? 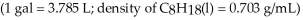
Definitions:
Management by Objectives
A performance management approach where employees and managers collaboratively set, monitor, and achieve specific objectives within a predefined timeframe.
Desired Outcome
The specific result or achievement that a person or organization aims to reach through their actions or a strategic plan.
Distinguishing Characteristic
A unique or defining trait, quality, or feature that sets an individual, group, or object apart from others.
Personal Contact
involves direct interaction between individuals, usually face-to-face, for communication or relationship building.
Q4: Given the bond enthalpies C-O (360),C =O
Q6: Liquid mercury freezes at a temperature of
Q21: A balloon with volume 750 cm<sup>3</sup> is
Q22: Choose the INCORRECT statement.<br>A)Bonds between like atoms
Q51: Reactive metals such as iron will reduce
Q54: Methane and oxygen react to form carbon
Q64: Why was Moseley so sure from his
Q87: The core electrons are called valence electrons.
Q103: How many moles of Co<sup>2+</sup> (aq)are present
Q167: The chemical reaction occurring during the discharge