The complete combustion of octane,a component of gasoline,is represented by the equation:
2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(l)
How many liters of CO2(g) ,measured at 63.1 °C and 688 mmHg,are produced for every gallon of octane burned? 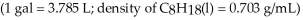
Definitions:
Contract
A legally binding agreement between two or more parties that is enforceable by law.
Environmental Performance
Refers to an organization’s impact on the environment, including the ways in which it manages resources and waste to mitigate harmful effects.
Cost Reductions
Strategies and actions taken to lower expenses and improve efficiency.
Materials Recovery Programs
Initiatives or practices aimed at collecting, processing, and repurposing materials to prevent waste and conserve resources.
Q2: Convert to the equivalent pressure in atmospheres,
Q5: Calculate the height in meters of a
Q22: For the molecule <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="For the
Q22: What is the concentration of FeCl<sub>3</sub> in
Q62: How many grams of AgNO<sub>3</sub> are needed
Q69: Why is the electron affinity slightly positive
Q95: Gases tend to behave ideally at:<br>A)low temperature
Q96: The substance BaCl<sub>2</sub> is a:<br>A)salt<br>B)weak acid<br>C)strong base<br>D)weak
Q100: Which statement is correct for the structure
Q141: If the density of ethanol,C<sub>2</sub>H<sub>5</sub>OH,is 0.789 g