Using the heat of combustion of methanol as -726.6 kJ/mol and the following data: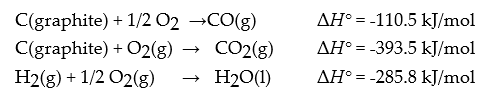
Determine ΔrH° for the following reaction:
CO(g) + 2 H2(g) → CH3OH(l)
Definitions:
Zero-Coupon Bonds
Bonds that do not pay periodic interest payments and are issued at a deep discount, maturing at face value.
STRIPS
Separate Trading of Registered Interest and Principal Securities, a type of U.S. government security that allows the separate trading of interest and principal components.
Treasury Notes
Intermediate-term U.S. government debt security with a maturity of 1 to 10 years and pays interest every six months.
Maturity at Issue
The predetermined date when a financial instrument, such as a bond, will come due and the principal is to be paid back to investors.
Q19: How many millilitres of 0.132 mol L<sup>-1</sup>
Q27: In the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="In the
Q36: If someone were to light a cigar
Q45: How many liters of H<sub>2</sub> are needed
Q50: Choose the INCORRECT statement concerning electromagnetic radiation.<br>A)Infrared
Q56: Cryolite is a compound needed for the
Q81: The actinides refer to what group of
Q116: Calculate Δ<sub>f</sub>H° of octane,C<sub>8</sub>H<sub>18</sub>(l),given the enthalpy of
Q136: How many grams of H<sub>3</sub>PO<sub>4</sub> are in
Q140: Calculate the number of grams of solute