Given the heat of formation of the following compounds: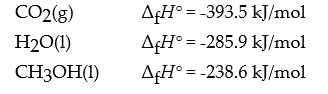
What is the value of ΔrH° for the reaction:
CH3OH(l) + 3/2 O2(g) → CO2(g) + 2 H2O(l)
Definitions:
Total Utility
The total satisfaction or benefit obtained from consuming a given quantity of goods or services.
Marginal Utility
The added satisfaction or utility that a consumer derives from consuming an additional unit of a good or service.
Total Utility
This term refers to the total satisfaction received by consuming a certain amount of goods or services.
Milk Shakes
Although commonly a beverage, if not directly relevant to economics or provided as a specific economic term, the answer is NO.
Q14: Given the bond enthalpies C-C (348),C=O (707),O
Q16: After drawing the Lewis dot structure for
Q33: The three molecular shapes an sp<sup>3</sup> hybridized
Q45: Iron in the form FeCl<sub>2</sub> can be
Q63: Balance the following equation in basic solution:<br>Cr<sup>3+</sup>
Q64: After drawing the Lewis dot structure of
Q74: Sodium metal reacts with water to produce
Q86: The phenomenon of supercooling refers to the
Q86: Enthalpy is an extensive property.
Q113: Convert 421 kPa to the equivalent pressure