When 1.50 mol of CH4(g) reacts with excess Cl2(g) at constant pressure according to the chemical equation shown below,1062 kJ of heat are released.Calculate the value of ΔH for this reaction,as written.
2 CH4(g) + 3 Cl2(g) → 2 CHCl3(l) + 3 H2(g) 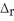 H° = ?
H° = ?
Definitions:
Follower Characteristics
The attributes or qualities of individuals who support and are guided by a leader.
Workflow
The sequence of processes through which a piece of work passes from initiation to completion.
Authority Relationships
These are the frameworks within which power, responsibilities, and duties are distributed among individuals or groups, typically in an organizational setting.
Role-Centered
Focusing on the responsibilities and expectations associated with a particular social or professional position.
Q9: Choose the INCORRECT statement about NO<sub>2</sub><sup>+</sup>.<br>A)There is
Q10: Automotive air bags inflate when sodium azide
Q19: An automobile tire at 32.0 psi at
Q27: In the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="In the
Q28: Choose the groups of molecules below in
Q41: The existence of discrete (quantized)energy levels in
Q44: At 1.0133 bar,the heat of sublimation of
Q56: According to the kinetic-molecular theory of gases:<br>A)gaseous
Q59: How many electrons are in the outermost
Q107: Which of the following is diamagnetic?<br>A)N<sub>2 </sub><br>B)O<sub>2</sub><sup>-