In the presence of excess oxygen,methane gas burns in a constant-pressure system to yield carbon dioxide and water:
CH4(g) +2O2 (g) → CO2(g) + 2H2O (l) 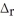 H = -890.0 kJ
H = -890.0 kJ
Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.
Definitions:
Performance Management
The process of ensuring employees' activities and outputs align with the organization's goals, through planning, monitoring, and reviewing employee performance.
Operations Team
A group within an organization responsible for overseeing and managing the day-to-day activities required to maintain and enhance the business's operational functions.
Recruitment and Selection
The process of identifying, attracting, interviewing, selecting, hiring, and onboarding employees. It involves evaluating candidates' suitability for a specific role or organization.
Workforce Planning
The process of analyzing, forecasting, and planning workforce supply and demand to meet the organization's objectives.
Q1: When 11.0 g of calcium metal is
Q1: Which of the following represents a disproportionation
Q12: In the reaction: 4 Zn + K<sub>2</sub>Cr<sub>2</sub>O<sub>7</sub>
Q19: Given the following bond lengths in pm:<br>C-C,154;C
Q34: Sulfuric acid can be prepared by a
Q51: Given the bond enthalpies C =C (611),C
Q68: How many liters of gas is 10.8
Q99: A 4.0 L sample of N<sub>2</sub>(g)at 760
Q105: What reagent could not be used to
Q108: Which of the following gases has the