When 2.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere,the reaction shown below occurs and the temperature of the resulting solution rises from 22.00 °C to 40.32 °C.If the specific heat of the solution is 4.18 J g-1°C-1 ,calculate 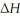 For the reaction,as written.
For the reaction,as written.
Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g)
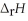 = ?
= ?
Definitions:
Career Summary
A brief explanation of an individual's professional background, including significant achievements and experiences.
Extensive Reasons
Detailed and thorough explanations or justifications for an action, belief, or decision.
Regular Customer
A customer who frequently purchases products or services from the same business, showing loyalty and consistency in their patronage.
Complete Refund
The process of giving back the full amount of money paid by a customer for a product or service, often due to dissatisfaction or a defect.
Q17: A 50.0 mL canister of Freon-12 (CF<sub>2</sub>Cl<sub>2</sub>)was
Q30: A titration is a method of adding
Q40: A 10.0 L container of unknown gas
Q68: When an electron goes from a high
Q68: A solution of potassium permanganate was standardized
Q79: The quantum numbers of the last electron
Q91: After drawing the Lewis dot structure for
Q95: Arrange the following compounds in order of
Q110: The chemical reaction occurring during the discharge
Q113: When the following equation is completed and