When 50.0 mL of 0.400 mol L-1 Ca(NO3) 2 is added to 50.0 mL of 0.800 mol L-1 NaF,CaF2 precipitates,as shown in the net ionic equation below.The initial temperature of both solutions is 23.0 °C.Assuming that the reaction goes to completion,and that the resulting solution has a mass of 100.00 g and a specific heat of 4.18 J g-1°C-1 ,calculate the final temperature of the solution.
Ca2+(aq) + 2 F-(aq) → CaF2(s) 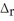 H = -11.5 kJ
H = -11.5 kJ
Definitions:
Cross-Cultural Teams
Groups comprising members from diverse cultural backgrounds, working together to achieve common objectives.
Attitudes Toward Hierarchy
The way individuals perceive and react to the levels of structure and authority within an organization or society.
Marketing Advantage
The edge a company gains over its competitors by offering greater customer value, either through lower prices or by providing more benefits that justify higher prices.
Q4: How many grams of H<sub>2</sub> gas can
Q30: Oxygen gas at 34.5 °C is compressed
Q44: Which of the following has the most
Q61: What is the wavelength of a bullet
Q62: An expansion of gas by a system
Q68: Calculate the enthalpy change for the following
Q68: How many liters of gas is 10.8
Q81: In an acid-base neutralization reaction,38.74 mL of
Q88: The Bohr theory explains that an emission
Q90: Halothane is one of the modern anesthetics