Using the heat of combustion of methanol as -726.6 kJ/mol and the following data: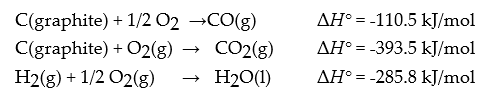
Determine ΔrH° for the following reaction:
CO(g) + 2 H2(g) → CH3OH(l)
Definitions:
Cost Of Goods Sold
Cost of goods sold (COGS) is the direct cost attributable to the production of the goods sold by a company, including the cost of materials and labor.
Gross Profit
The financial gain a company achieves after subtracting the cost of goods sold from its total revenue.
Operating Expenses
Expenses incurred in the normal operation of a business, excluding the cost of goods sold.
Net Sales
This is the amount of sales generated by a company after deducting returns, allowances for damaged or missing goods, and any discounts allowed.
Q4: The standard heat of formation of solid
Q8: Bonds between identical atoms are nonpolar bonds.
Q21: Based on the balanced chemical equation shown
Q21: How many σ- and π-bonds,respectively,are there in
Q53: Choose the INCORRECT statement.<br>A)Molecules with all paired
Q62: An expansion of gas by a system
Q76: You have three cylinders containing O<sub>2</sub> gas
Q79: Compute Δ<sub>r</sub>H° for the following reaction.The value
Q84: The heat of combustion of several fuels
Q99: How many bond pairs ("bp")and how many